Michigan State University College of Human Medicine
Henry Ford Jackson Hospital
Jackson, Mich.
Department of Anesthesiology
Emory University School of Medicine
Atlanta, Ga.
Department of Anesthesiology and Pain Management
The University of Texas Southwestern Medical Center
Dallas
Department of Anaesthesiology, Critical Care and Pain
Tata Memorial Hospital
Mumbai, India
Anesthesiologists are well versed in managing anatomically difficult airways. The American Society of Anesthesiologists’ Difficult Airway Algorithm has been tested repeatedly and forms the backbone of daily airway management in the OR.1 Having focused decades’ worth of research and training on anatomically difficult airways, we have subsequently come to define and understand a new group of patients whose morbidity and mortality associated with airway management remains exceedingly high: patients with a physiologically difficult airway.
The term “physiologically difficult airway” (PDA) was coined by Jarrod Mosier in 2015, to better describe patients whose existing critical illness places them at high risk for intubation-related complications, despite having an uncomplicated and simple tracheal intubation.2 The concept of a PDA continues to evolve from this original description, helping to guide research and best practice for this high-acuity population.3
Tracheal Intubation in Critically Ill Patients: The Riskiest Procedure We Perform
Performing tracheal intubation in patients compromised by critical illness remains a procedure with an extremely high associated morbidity and mortality. The most comprehensive data on the subject were published in 2021 by Russotto et al, in which researchers evaluated almost 3,000 critically ill patients requiring intubation in 29 different countries.4 The data showed that major adverse events occurred in 45% of critically ill patients within 30 minutes of tracheal intubation. The most common adverse event was cardiovascular instability, seen in 42.6% of patients.4 Cardiac arrest occurred in 3.1% of patients.
The second most common adverse event was severe hypoxemia, defined as oxygen saturation (SpO2) less than 80%, which was seen in 9.3% of patients. Similar incidences of adverse events were found in other studies of airway management in physiologically difficult airways, further emphasizing the high morbidity and mortality of this procedure.
Who Is at Risk for a Physiologically Difficult Airway?
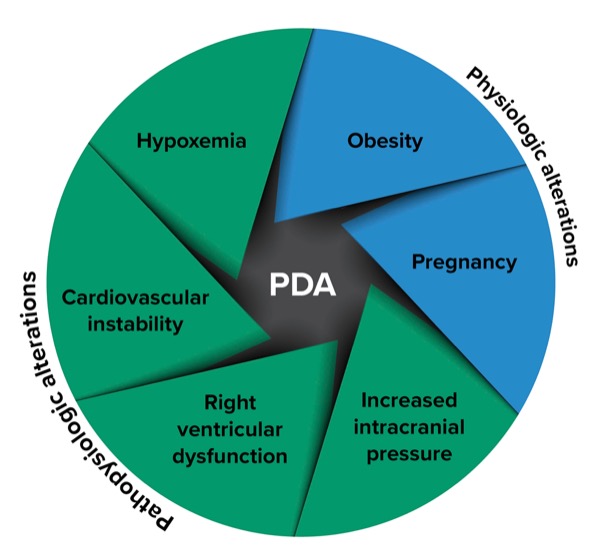
When the PDA was first described by Mosier et al, they identified four specific types: hypoxemia, hypotension, severe metabolic acidosis and right ventricular failure.2 Each of these patient populations presents its own unique set of challenges related to induction, intubation and introduction of positive pressure ventilation.
More recently, a Delphi study of experts in PDA management expanded on these original groups to include patients with pathophysiologic changes associated with their critical illness (including hypoxemia, cardiovascular instability, right ventricular dysfunction and increased intracranial pressure), as well as patients with physiologic alterations that occur in obesity and pregnancy (Figure).5
Optimizing Oxygenation Before, During And After Tracheal Intubation
Large prospective observational studies have consistently identified hypoxemia as the second most common complication of tracheal intubation in critically ill adults and/or those with a PDA.4,6 Preoxygenation with tidal or vital capacity breaths and 100% fraction of inspired oxygen (FiO2) has long been a mainstay of airway management in the OR. However, patients with a PDA typically will have some degree of ventilation-to-perfusion mismatch and/or shunt physiology, wherein perfused alveolar units are not ventilated. These pathophysiologic changes serve to diminish the extent to which the partial pressure of oxygen (PaO2) increases relative to the FiO2.
Positive pressure is required to facilitate alveolar recruitment and meaningfully extend the safe apneic interval, especially in patients with more severe shunt physiology. Common examples include pneumonia, acute pulmonary edema and acute respiratory distress syndrome. Patients with these conditions are at risk for hypoxemia during tracheal intubation and require modified approaches to optimize oxygenation. Over the past 20 years, a body of literature has emerged to help inform best practices and synthesize an evidence-based approach to the mitigation of hypoxemia during tracheal intubation. Fundamentally, there are three potential approaches to consider both in isolation and in combination with one another: high-flow oxygen delivery via nasal cannula (HFNC), noninvasive positive pressure ventilation (NIPPV) and ventilation via bag-valve-mask (BVM).
In contrast to other approaches, HFNC has the practical and conceptual benefit of application before and during tracheal intubation. HFNC with sufficiently robust flows can deliver a high FiO2, generate a non-zero amount of continuous positive airway pressure (CPAP), and potentially facilitate apneic oxygenation during tracheal intubation without obstructing the airway operator. In meta-analyses examining preoxygenation methods in critically ill adults, HFNC has variably been suggested or demonstrated to reduce the odds of hypoxemia during tracheal intubation compared with so-called conventional oxygen therapy (i.e., preoxygenation with a face mask).7-9 Varying results among meta-analyses are probably attributable to both the examination and inclusion of additional trials over time and a clinically plausible degree of treatment effect heterogeneity. HFNC’s relative efficacy as a preoxygenation strategy is likely diminished in patients with moderate or severe respiratory failure and a P/F (PaO2/FiO2) ratio of 200 or lower owing to a mismatch between the modest degree of CPAP afforded relative to worsening shunt physiology.10-14
When examined by meta-analysis, NIPPV consistently demonstrated a reduction in the odds of hypoxemia during tracheal intubation compared with both conventional oxygen therapy and HFNC.7-9 This finding is both physiologically and clinically plausible considering that a mask interface affords more precise control of FiO2 (with less entrainment of room air) and delivery of higher airway pressures compared with HFNC, thus accomplishing more robust alveolar recruitment. In comparison with HFNC, NIPPV is often more economical and consumes less oxygen at any given FiO2, which is potentially advantageous in resource-limited environments. The clinical benefits of NIPPV are perhaps most pronounced in patients with a P/F ratio of 200 or lower. When selecting between HFNC and NIPPV an adaptive approach is advisable15:
- when the P/F is > 200, HFNC or NIPPV;
- when the P/F is = 200 but > 100, NIPPV with lower airway pressures; and
- when the P/F is = 100, NIPPV with higher airway pressures.
Additionally, the combination of NIPPV and HFNC is possible (e.g., by applying a mask over the HFNC interface) and conceptually affords particularly at-risk patients the best of both approaches.16
In view of this growing body of evidence, which now includes the recent high-profile publication of the PREOXI trial, guidelines and expert consensus now support the use of HFNC and/or NIPPV to optimize oxygenation before and during tracheal intubation in adults with a PDA.5,14,17
While the selection of a preoxygenation strategy is of critical importance, it is important to remember that it is just one element of an overall approach intended to optimize oxygenation. An ideal approach would also focus on all steps that can optimize oxygenation before, during and after tracheal intubation.18 For example, apneic and/or rescue ventilation with BVM has been demonstrated to be both safe and effective in preventing oxygen desaturation during tracheal intubation and is another key component of a more holistic approach.19,20 It is also worth noting that repeated attempts at laryngoscopy have been consistently associated with increased risk for hypoxemia.4,6,21 As such, interventions that improve the odds of first-pass success, which are discussed below, also should be seen as key elements of optimizing oxygenation.
Hemodynamic Optimization
In the INTUBE (International Observational Study to Understand the Impact and Best Practices of Airway Management in Critically Ill Patients) study, researchers found that cardiovascular instability was the most common complication associated with airway management outside of the OR, occurring in 43% of critically ill patients.4 As expected, patients who experienced complications (including cardiovascular instability and cardiac arrest) had a higher 28-day mortality.4 Several factors contribute to the higher incidence of cardiovascular collapse during tracheal intubation in critically ill patients versus those in the OR. These factors include the underlying critical illness (e.g., sepsis, hypovolemia, vasoplegia, cardiac dysfunction), medications and dose used to facilitate intubation (e.g., propofol, benzodiazepines, opioids), loss of sympathetic drive, and the transition to positive pressure ventilation following intubation.22
The high incidence of cardiovascular collapse associated with tracheal intubation in critically ill patients and its effect on mortality underscore the need to focus on prevention rather than merely treating the complication once it occurs. It also highlights the importance of close peri-procedural hemodynamic monitoring in these patients. A recent Delphi consensus study on the PDA emphasized that minimum mandatory monitoring during tracheal intubation in these patients should include noninvasive blood pressure, continuous electrocardiography and pulse oximetry.5 Point-of-care ultrasound (POCUS) can help identify patients at risk for cardiovascular collapse, identify etiologies of shock, and provide real-time hemodynamic and respiratory assessment, which may aid in physiologic optimization.5,23 Experts recommended the use of peri-procedural POCUS, when feasible, to improve the safety of tracheal intubation in this high-risk population.5
Fluid Bolus and Vasopressors
The administration of a fluid bolus and early use of vasopressors have been recommended to reduce the incidence of cardiovascular collapse during tracheal intubation.18,24 Two multicenter randomized trials conducted in North America compared the administration of a 500-mL IV saline bolus with no fluid bolus in a heterogeneous group of critically ill adult patients prior to tracheal intubation. Both studies showed no reduction in the overall incidence of cardiovascular collapse following intubation. The PrePARE (Preventing Cardiovascular collaPse With Administration of Fluid Resuscitation Before Endotracheal Intubation) study was conducted in an unselected patient population, whereas the PrePARE II study involved patients who received positive pressure ventilation between induction and laryngoscopy.25,26 Given these findings, the use of a fluid bolus in these settings may be considered on a case-by-case basis, potentially guided by an assessment of fluid responsiveness, if feasible.
Whether specific subgroups benefit from a fluid bolus prior to tracheal intubation or from the use of pre-intubation vasopressors to reduce the incidence of cardiovascular collapse remains to be determined.22 Two ongoing international trials, the FLUVA Trial (ClinicalTrials.gov Identifier: NCT05318066) and the PREVENTION Trial (NCT05014581), are currently investigating the effectiveness of the preemptive administration of norepinephrine in reducing the incidence of cardiovascular collapse in critically ill adults undergoing tracheal intubation. The results of these trials are expected to provide more definitive answers. In the meantime, expert consensus supports hemodynamic optimization prior to tracheal intubation in this high-risk population. Optimization includes the use of vasopressors and/or inotropes as needed to prevent or reduce the risk of peri-intubation cardiovascular collapse.5
Performing the Procedure
Patient Positioning
Patient positioning is perhaps the first step in the performance of tracheal intubation, and optimal positioning is essential to increase the success of intubation and avoid complications. The objective should be to optimize both the anatomic and physiologic parameters. The two most used positions are the “sniffing position” and the “ramped position.” The sniffing position (achieved by aligning the oropharyngeal–laryngeal axes) may make glottic visualization and tracheal intubation easier. The ramped position (keeping the external auditory meatus level with the sternal notch) prevents reduction in functional residual capacity, improves preoxygenation and may reduce the risk for pulmonary aspiration.
The superiority of one versus the other is debatable27 and provider training may have a significant impact on the success of tracheal intubation related to patient positioning.28-30 In addition, patient characteristics may influence the choice of appropriate position. While a patient with a PDA would significantly benefit from a ramped position, a patient with an anatomically difficult airway may not. Hence, patient positioning should perhaps be tailored to patient characteristics, as well as the training history of the intubating provider. Experts in management of PDA recommend a head-elevated laryngoscopic position (also known as the semi-Fowler position) with the head of the bed elevated to 30 degrees, for tracheal intubation in patients with a PDA.5
Rapid Sequence Intubation
A rapid sequence intubation (RSI) or modified RSI technique is commonly used in patients who need emergent tracheal intubation. While there is no agreed definition of RSI or modified RSI in the literature, RSI was designed to facilitate prompt establishment of a secure airway in patients at high risk for aspiration. The main objective of this technique is to minimize the time interval between loss of protective airway reflexes and securing the airway. Despite the technique’s widespread use, there is still no consensus on the optimal components and sequence of an RSI. Applying cricoid pressure during RSI has long been debated. Although cricoid pressure may be effective for occlusion of the upper esophagus, its clinical benefits are unproven.31,32 It has been proposed that cricoid pressure (if applied) should be released in case of difficulty with visualization of the vocal cords during tracheal intubation.5
Device Selection
Since the rates of complications increase with the number of attempts at tracheal intubation, first-pass success is paramount.33,34 Tracheal intubation in critically ill patients is technically more challenging than elective intubation performed in the OR. It has been observed that the same patients who are intubated in the OR for elective surgery, who subsequently require emergent intubation in the ICU later during their hospital admission tend to have a worse glottic visualization, a lower first-pass success rate, and a higher incidence of complications during their intubation in the ICU.35 It is important to use tools and strategies that can improve first-pass intubation success, and the use of video laryngoscopy (VL) may be one of them.
While direct laryngoscopy (DL) affords the advantage of aligning the oropharyngeal and laryngeal axes better (therefore facilitating passage of the endotracheal tube), the advent of VL has made visualization of the glottic opening easier. A recent systematic review and meta-analysis that compared VL and DL for intubation in emergency department and ICU patients demonstrated that VL was associated with higher rates of first-pass success, reduced rates of failed tracheal intubation, reduced complications and improved glottic visualization.36 The use of VL may also be associated with reduced rates of dental injuries and aspiration events. However, there was no difference in mortality between the two techniques.
Experts in PDA management also recommend that VL should be available during the management of a PDA and be routinely employed during the first attempt at tracheal intubation where feasible.5 The use of airway adjuncts, such as the stylet or bougie, to facilitate tracheal intubation varies across clinical settings. The STYLETO (Stylet for Orotracheal intubation) trial demonstrated that using a stylet during tracheal intubation with DL resulted in a significantly higher first-pass success rate.37 Another study comparing the use of a bougie and a stylet in critically ill adults undergoing tracheal intubation reported no difference in the incidence of first-pass success with the use of a bougie versus use of a tracheal tube with stylet.38 Taken together, these studies highlight the importance of routinely using a stylet or a bougie during the first attempt, rather than as a rescue, to improve initial success.
Sedative–Hypnotic Induction Drugs
Tracheal intubation in critically ill patients routinely involves the use of hypnotic agents and neuromuscular blocking agents to facilitate insertion of the endotracheal tube. The pharmacologic management of the PDA continues to evolve. The optimal use and choice of pharmacologic agents should be guided by each patient’s unique situation and comorbidities.
Drugs used for induction of anesthesia for tracheal intubation can increase the risk for hemodynamic complications. The appropriate choice and dosing of such agents is critical to prevent peri-intubation cardiovascular collapse. Although propofol provides superior conditions for tracheal intubation, it may not be suitable in most critically ill patients. A post hoc analysis of the INTUBE study showed that the use of propofol for induction was the only modifiable independent predictor of cardiovascular collapse in these patients.39 Although the mean dose of propofol used in the INTUBE study was significantly lower than standard anesthetic induction doses (1 vs. 2 mg/kg, respectively), the association of propofol with subsequent cardiovascular collapse appeared to be independent of the dose used. In this light, it has been recommended that standard anesthetic induction doses of propofol should be avoided in patients with a PDA to decrease the risk for peri-intubation hemodynamic collapse.5
Experts recommend using sedative–hypnotic drugs that have a more stable hemodynamic profile, such as ketamine and etomidate, for tracheal intubation in patients with a PDA.5 Trials comparing these two drugs for tracheal intubation in critically ill adults have been inconclusive.40-42 A recent retrospective study43 and one Bayesian meta-analysis44 showed that induction with etomidate may be associated with a higher risk for hospital mortality than ketamine. This is hypothesized to be due to the well-known adrenal suppression associated with an induction dose of etomidate. A more recent meta-analysis demonstrated that ketamine probably results in more hemodynamic instability during the peri-intubation period compared with etomidate, and appears to have no effect on first-attempt success or hospital mortality.45 Hence, the decision to use either of these drugs should be based on individual patient characteristics, as well as local practices.
Drug admixtures such as propofol combined with ketamine (i.e., ketofol) and propofol combined with etomidate may have favorable hemodynamic profiles,46 but the admixture ratios are not well defined, standardized or approved.
Neuromuscular Blockade
Use of neuromuscular blockade during tracheal intubation is associated with greater first-pass success and should be considered for all patients.47 Many studies and meta-analyses comparing succinylcholine and rocuronium for tracheal intubation in critically ill patients have failed to demonstrate any clinically meaningful difference between the two drugs, and it has been recommended that either could be used for facilitating tracheal intubation in patients with a PDA.5 However, succinylcholine should be avoided in certain situations, such as patients with skeletal muscle myopathies, known allergy, history of malignant hyperthermia, hyperkalemia and significant burn injury. Sugammadex may be used for the rapid reversal of rocuronium in an emergency, but there is limited safety data for its use in critically ill patients.
Post-Intubation Care
Confirmation of Endotracheal Tube Placement
Inadvertent esophageal intubation continues to occur in approximately 5% of patients with a PDA.4 Although waveform capnography is considered the gold standard for confirmation of endotracheal tube placement, the INTUBE study demonstrated that only 25% of intubations used waveform capnography. The same data set showed that 58% of intubations utilized auscultation for confirmation of endotracheal intubation. The Project for Universal Management of Airways now recommends continuous waveform capnography for verifying the presence of sustained exhaled carbon dioxide, requiring four criteria to be met48:
- amplitude rising during exhalation and falling during inspiration;
- consistent or increasing amplitude over at least seven breaths;
- peak amplitude more than 1kPa (7.5mm Hg) above baseline; and
- the reading is clinically appropriate.
Experts in PDA management agreed on the importance of demonstrating sustained exhaled carbon dioxide for seven consecutive breaths to confirm correct endotracheal tube placement.5 They also endorsed chest auscultation, chest X-ray and/or bronchoscopy as acceptable methods of confirming optimal depth of the tube in the trachea.
Post-Intubation Ventilator Settings
Immediately following confirmation of endotracheal tube placement, attention must be focused back to management of any physiologic compromise that has ensued during intubation and transition to positive pressure ventilation. Although patients with PDA represent diverse pathophysiology, experts recommend using lung-protective ventilation for all, with target tidal volumes of 6 to 8 mL/kg of ideal body weight.5
Mitigating the Risk for Awareness With Paralysis
Given the widespread use of nondepolarizing neuromuscular blockade at doses compatible with RSI, it is reasonable to expect a period of paralysis for at least 45 minutes following induction, depending on both patient and drug-specific factors. Experts recommend infusions of sedative medications to prevent awareness, using clinical assessment of sedation depth to guide infusion dosing.5
Although post-intubation sedation is recommended and indeed used widely, awareness with paralysis presents a serious concern. As sugammadex has become more widely available for rescue reversal in a “can’t intubate, can’t oxygenate” scenario, use of rocuronium in emergent intubations has increased.49
In the ED-AWARENESS study, investigators conducted a prospective, observational study of patients requiring emergent intubation in the emergency department.50 Of the 383 patients, 7% of those who underwent RSI reported memories of awake paralysis. After careful clinical review, the true incidence of awake paralysis was deemed to be around 2.6%. The investigators found that exposure to rocuronium during RSI was a significant predictor of subsequent awareness with paralysis.50 A second prospective trial enrolled over 800 patients requiring intubation in the emergency department.51 They found that 9% of patients had potential awareness with paralysis, and 4% of patients had potential awareness during laryngoscopy itself. Notably, exposure to induction agents and post-intubation sedative infusions varied widely in both studies.
More research is needed to define optimal induction doses of hypnotic agents in the setting of critical illness, as well as to investigate the role of awareness monitors (such as processed electroencephalogram) to prevent awareness with paralysis in this patient population.
Human Factors
Human factors play an integral role in the safe performance of tracheal intubation in patients with a PDA. While it is important to optimize the patient to tolerate the procedure with minimal physiologic perturbations, it is equally important to prepare the team that is involved in the performance of the procedure. A recent international Delphi process identified various opportunities to help optimize the team for success during the management of a PDA.5 One of the key interventions is the use of checklists. The use of checklists has long been regarded by various industries as a method to decrease cognitive load and reduce errors in stressful situations, and their use has now become ubiquitous in healthcare.52 Although the effectiveness of such checklists in reducing adverse outcomes in patients with a PDA is conflicting,53,54 the overall use of airway management checklists has been increasing.
Implementing a checklist for the management of a PDA can help ensure that necessary preparations and precautions have been taken, while simultaneously reducing cognitive overload and allowing for better decision-making. An appropriately designed intubation checklist that addresses equipment, drugs, team roles/composition, patient optimization, and both primary and backup plans for airway management can reduce errors of omission and may improve patient outcomes during the management of a PDA.5
Team Composition
The composition of the team that is involved in the management of a PDA may differ based on the hospital setting, clinical urgency, availability and skill set of team members, and available resources. As a result, the recommendations for the composition of the airway team also vary. While the Difficult Airway Society guidelines recommend a minimum of four and up to six staff members for performing tracheal intubation in critically ill adults,55 the PDA Delphi experts recommend that an intubation team should consist of at least three healthcare providers, including two airway operators, at least one of whom should have experience with airway management in critically ill patients.5
The definition of an experienced airway operator is unclear. It is undetermined whether the years of experience with tracheal intubation or the number of procedures performed should be prioritized. Also, it remains unclear whether dedicated training in critical care medicine should be a prerequisite for defining an experienced airway operator for managing a PDA.
These inconsistencies may reflect the multitude of definitions in the literature and the wide variation in the training curricula of ICU practitioners internationally. It is important that training requirements for providers performing airway management in patients with a PDA are well defined. The CASCADE (Curriculum for Airway Skills in Critically Ill Adults: a Delphi Evaluation) trial,56 currently underway, should help define a training curriculum for the management of a PDA.
In addition to team composition, emphasis should be placed on team dynamics, considering the situational challenges surrounding the management of a PDA. Elucidating the skill level of available staff members and establishing clear roles and expectations are essential elements of successful intervention. Similarly, closed-loop communication is essential in such stressful situations to prevent medical errors. Furthermore, ensuring a shared mental model among team members, discussion of primary and rescue plans, gathering and interpreting information, and anticipating problems are important tasks that can improve team performance and eventually improve patient outcomes.
Conclusion
Tracheal intubation of patients with a PDA continues to be associated with a high incidence of complications, including cardiovascular collapse and hypoxemia. The evidence supporting management of patients with a PDA continues to grow, helping to optimize techniques in peri-procedural oxygenation, prevention of hemodynamic collapse, first-pass success, management of post-intubation physiologic changes and ensuring optimal team performance. Implementation of these evidence-based techniques may help to achieve better outcomes in this high-acuity patient population.
References
- Anesthesiology. 2022;136(1):31-81.
- West J Emerg Med. 2015;16(7):1109-1117.
- Curr Opin Anaesthesiol. 2022;35(2):115-121.
- JAMA. 2021;325(12):1164-1172.
- Intensive Care Med. 2024;50(10):1563-1579.
- Crit Care Med. 2024;52(5):786-797.
- Lancet Respir Med. 2025 Mar 20.
- Crit Care Med. 2020;48(4):571-578.
- Crit Care. 2019;23(1):319.
- Lancet Respir Med. 2019;7(4):303-312.
- Sci Rep. 2020;10(1):3541.
- Intensive Care Med. 2020;46(12):2238-2247.
- Eur J Anaesthesiol. 2022;39(5):463-472.
- N Engl J Med. 2024;390(23):2165-2177.
- Indian J Anaesth. 2024;68(10):855-858.
- Intensive Care Med. 2016;42(12):1877-1887.
- Crit Care Med. 2023;51(10):1411-1430.
- Intensive Care Med. 2022;48(10):1287-1298.
- N Engl J Med. 2019;380(9):811-821.
- J Intensive Care Med. 2022;37(7):899-907.
- Crit Care. 2025;29(1):192.
- Anaesth Crit Care Pain Med. 2022;41(6):101158.
- Anesth Analg. 2023;137(1):124-136.
- Crit Care. 2015;19(1):257.
- Lancet Respir Med. 2019;7(12):1039-1047.
- JAMA. 2022;328(3):270-279.
- J Anaesthesiol Clin Pharmacol. 2019;35(3):289-291.
- Anesth Analg. 2016;122(4):1101-1107.
- Chest. 2017;152(4):712-722.
- Am J Emerg Med. 2017;35(7):986-992.
- Anesth Analg. 2022;135(5):1064-1072.
- Anesth Analg. 2023;136(2):e7.
- Intern Emerg Med. 2017;12(8):1235-1243.
- Acad Emerg Med. 2013;20(1):71-78.
- Anesthesiology. 2018;129(2):321-328.
- Crit Care Med. 2024;52(11):1674-1685.
- Intensive Care Med. 2021;47(6):653-664.
- JAMA. 2018;319(21):2179-2189.
- Am J Respir Crit Care Med. 2022;206(4):449-458.
- Lancet. 2009;374(9686):293-300.
- Emerg Med. 2023;65(5):e371-e382.
- Intensive Care Med. 2022;48(1):78-91.
- Am J Respir Crit Care Med. 2024;210(10):1243-1251.
- Crit Care. 2024;28(1):48.
- Crit Care Med. 2025;53(2):e374-e383.
- J Trauma Acute Care Surg. 2019;87(4):883-891.
- Ann Am Thorac Soc. 2015;12(5):734-741.
- Anaesthesia. 2022;77(12):1395-1415.
- Ann Emerg Med. 2015;65(4):363-370.e1.
- Ann Emerg Med. 2021;77(5):532-544.
- Chest. 2023;163(2):313-323.
- New Yorker. 2007 Dec 10:86-101.
- Intensive Care Med. 2010;36(2):248-255.
- Chest. 2018;153(4):816-824.
- Br J Anaesth. 2018;120(2):323-352.
- Curriculum for Airway Skills in Critically Ill Adults: a Delphi Evaluation (CASCADE). NCT06689748. Accessed June 2, 2025. https://clinicaltrials.gov/study/NCT06689748
Copyright © 2025 McMahon Publishing, 545 West 45th Street, New York, NY 10036. Printed in the USA. All rights reserved, including the right of reproduction, in whole or in part, in any form.
Download to read this article in PDF document:![]() Guidance for Managing the Physiologically Difficult Airway
Guidance for Managing the Physiologically Difficult Airway


Please log in to post a comment